Same Product, New Delivery
New locations for base-of-ear administration approved for BRD/foot rot treatment.
Pfizer Animal Health announces that two new, convenient, base-of-ear locations for subcutaneous administration have been approved in beef and dairy cattle for Excede® (ceftiofur crystalline-free acid) Sterile Suspension. These new techniques are in addition to the existing base-of-ear "toward the opposite eye" approved route of administration for all cattle, as well as the "middle third of the ear," which is approved in beef cattle and dairy heifers less than 20 months of age.
The two new techniques are a rostral direction, or toward the eye on the same side of the head, and a ventral administration, which is similar to a normal subcutaneous injection given at the base of the ear. The graphics below detail the two new techniques.
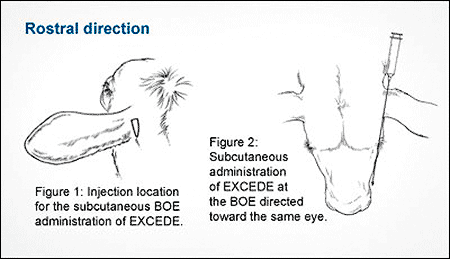
Hold the syringe and needle behind the ear to be dosed so the needle and syringe point in an imaginary line toward the animal's eye on the same side as the head.
See Figures 1 and 2.
Insert the needle through the loose skin at the BOE while maintaining the needle position. See Figure 2.
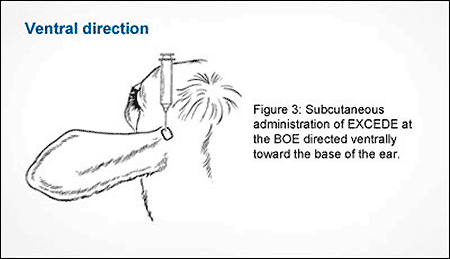
Hold the syringe and needle above the ear to be dosed so that the needle and syringe are pointing ventrally toward the base of the ear. See Figures 3 and 2.
Insert the needle through the loose skin at the BOE while maintaining the needle position. See Figure 3.
"I think veterinarians and producers will find the newly approved injection techniques to be more convenient than what we've previously had available for Excede," says Doug Hammon, senior manager, Cattle Technical Services, Pfizer Animal Health. "Producers should work with their veterinarian to determine which base-of-ear injection technique for Excede works best with their herd management protocols."
All approved administration locations provide the same 13-day meat withdrawal time and zero milk withholding following the last dose. The product also supports the tenets of Beef Quality Assurance and is backed by the Residue Free Guarantee™.*
To learn more about Excede and the newly approved base of ear administration locations, click here or contact your veterinarian or Pfizer Animal Health representative.
Important Safety Information: As with all drugs, the use of Excede is contraindicated in animals with known allergy to ceftiofur or to the ß-lactam group (penicillins and cephalosporins) of antimicrobials. Though safe in cattle when properly administered, inadvertent intra-arterial injection is possible and fatal. Excede has a preslaughter withdrawal time of 13 days following the last dose in cattle. Do not use in calves to be processed for veal.
*Residue Free Guarantee: If you use a Pfizer Animal Health-branded ceftiofur product according to label indications, and experience a violative ceftiofur milk or meat residue, Pfizer Animal Health will compensate you for the beef market value of the animal or purchase the tanker of milk at fair market value. You must purchase the product from a Pfizer Animal Health-approved supplier, use the product according to label indications, have documentation of the product purchase and treatment records, and have conducted training on appropriate use to ensure proper dose and route of administration of the product. Extra-label use as prescribed by a veterinarian is excluded from the guarantee. If you experience a ceftiofur residue violation after following label indications and the above steps, contact Pfizer Animal Health VMIPS (Veterinary Medical Information and Product Support) at 855-4AH-PFIZER (855-424-7349) to report the situation.
Editor's Note: This article was provided by Pfizer Animal Health.

[Click here to go to the top of the page.]











